
Thus one must determine which Electron Groups are from a σ bond, and thus associated with a peripheral atom, and which are not. The molecular geometry describes the relative positions of the atoms in the molecules. Once the Electron Group geometry is determined, one can finally predict the molecular geometry. Identify which Electron Groups are associated with bonds and determine the Molecular Geometry. The molecular geometry describes the arrangements of atoms around the central atoms and does not include the positions of lone pairs. Note! The Electron Group Geometry is NOT the Molecular Geometry.
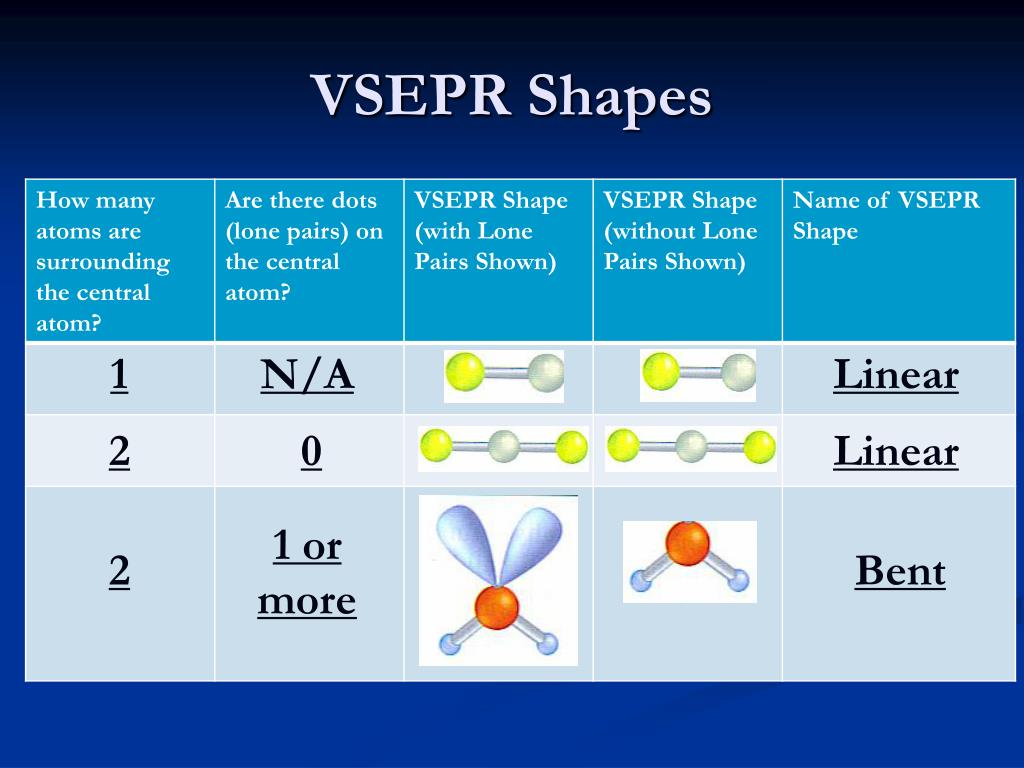
Thus the table accounts for all commonly encountered geometries. For all but the largest atoms (when bound to small groups), there is insufficient space to accommodate more than six Electron Groups around the central atom. The results are shown in the following table. With a knowledge of trigonometry and calculus, one can solve this minimization problem. The electron groups (lone pairs and σ bonding pairs) will assume whatever geometry minimizes their mutual repulsion (Coulomb's law). Once the number of Electron Groups ( N EG) is known, one can predict the geometry of the Electron Groups. Use N EG to determine the geometry of the Electron Groups. N EG = N LP + N σ Electron Group Geometry 3. For the purpose of determining the number of Electron Groups, an entire bond (single, double, or triple) counts as a one Electron Group. The number of Electron Groups equals the total number of lone pairs ( N LP) and σ bonds ( N σ) around the central atom. Pairs of electrons in π bonds do not count as Electron Groups in VSEPR. An Electron Group is a pair of electrons in a σ bond or a nonbonding pair. With a good Lewis structure in hand, identify the central atom (for water this is oxygen) and determine the number of Electron Groups around the central atom. Determine number of electron groups ( N EG) around the central atom. Also recall that a Lewis structure shows only the valence electrons (no core electrons).
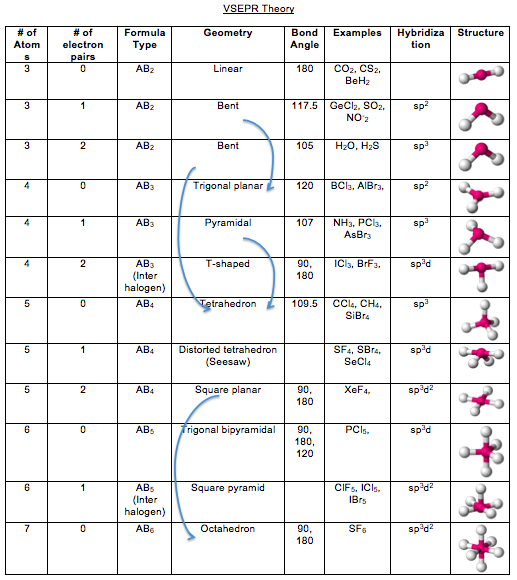
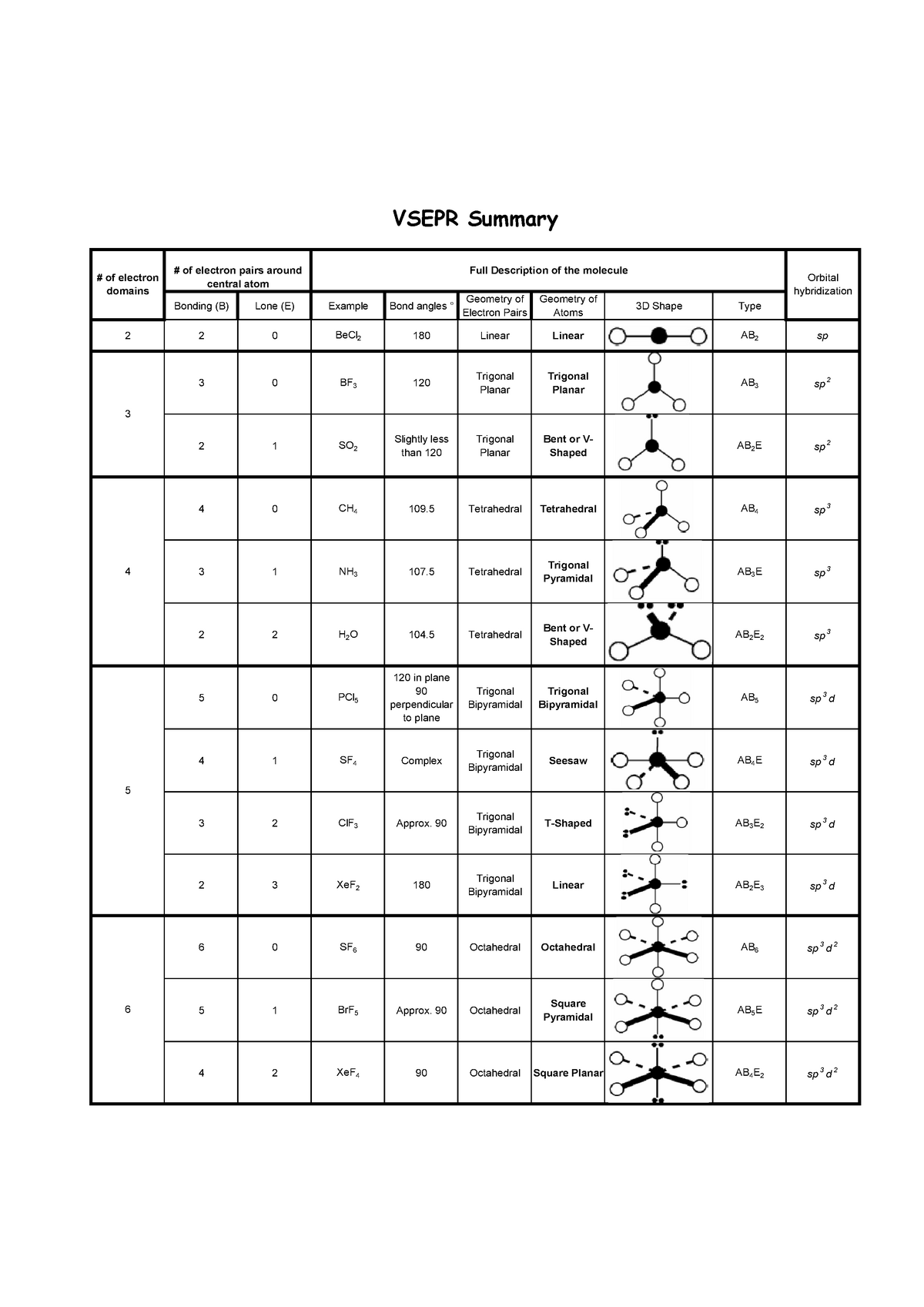
Thus the Lewis structure depicts electrons as pairs, either in bonds or as lone pairs (sometimes called nonbonding pairs). Most stable molecules contain an even number of electrons and the electrons occupy orbitals as pairs (one electron spin-up and the other spin-down). The starting point for the VSEPR Model is to determine the connectivity of the atoms and write a good Lewis structure for the molecule. Write the best Lewis structure for the molecule.

That is, there will not be a negative end and positive end of the molecule. If the water molecule is linear, the molecule will be non-polar. The lower part of the molecule will have a partial negative charge (because the oxygen strongly attracts shared electrons to itself), while the top part of the molecule will have a partial positive charge (because hydrogen atoms have a weaker pull than oxygen on shared electrons). If the water molecule has the shape of a V (with the oxygen at the lower vertex and hydrogens at the upper left and right), the molecule will be polar. The shape of a molecule has important implications for its properties and reactivity. The water molecule consists of an oxygen atom covalently bound to two hydrogen atoms.ĭo the atoms lie on a straight line (linear geometry)? Valence-Shell Electron-Pair Repulsion (VSEPR) Model Molecular Geometry


 0 kommentar(er)
0 kommentar(er)
